
Problem: Write the K sp expression for the following weak electrolytes: Mn(OH) 3 (s), Sr 3 (AsO 4 ) 2 (s), and Co 2 S 3 (s).Įxample: One liter of saturated calcium fluoride solution contains 0.0167 gram of CaF 2 at 25 o C. Ksp is constant at a given temperature (van’t Hoff equation) for a saturated solution of a given compound.

These expressions are called solubility product constant expressions because they involve the product of the equilibrium concentrations of the constituent ions, each raised to the power corresponding to the number of ions in the formula.
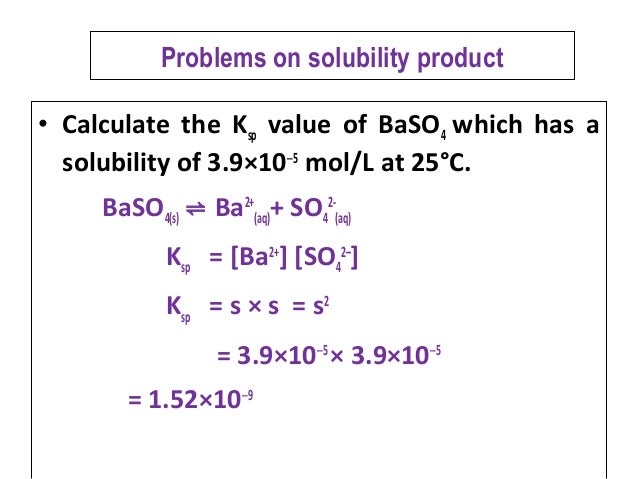
In general, M aX b(s) aM +b(aq) + bX -a(aq) is expressed as Ksp = a b Recall pure solids (and pure liquids) are not included in an equilibrium constant expression. The Equilibrium constant expression for this reaction can be written as: Ksp = When a reasonable quantity of solid BaSO4 is mixed with water, only a very small amount will dissolve to produce Ba +2 (aq) and SO 4 –2(aq)ġ.00 liter of saturated BaSO 4 solution will contain only ~0.0025 gram of dissolved BaSO 4. The expression is called the Solubility Product Constant (Ksp)Įxample: BaSO4 is a slightly soluble salt. Write and Equilibrium Constant Expression for a Slightly Soluble Salt: How do you know if a salt is soluble in water or not? Memorize the rules or ….
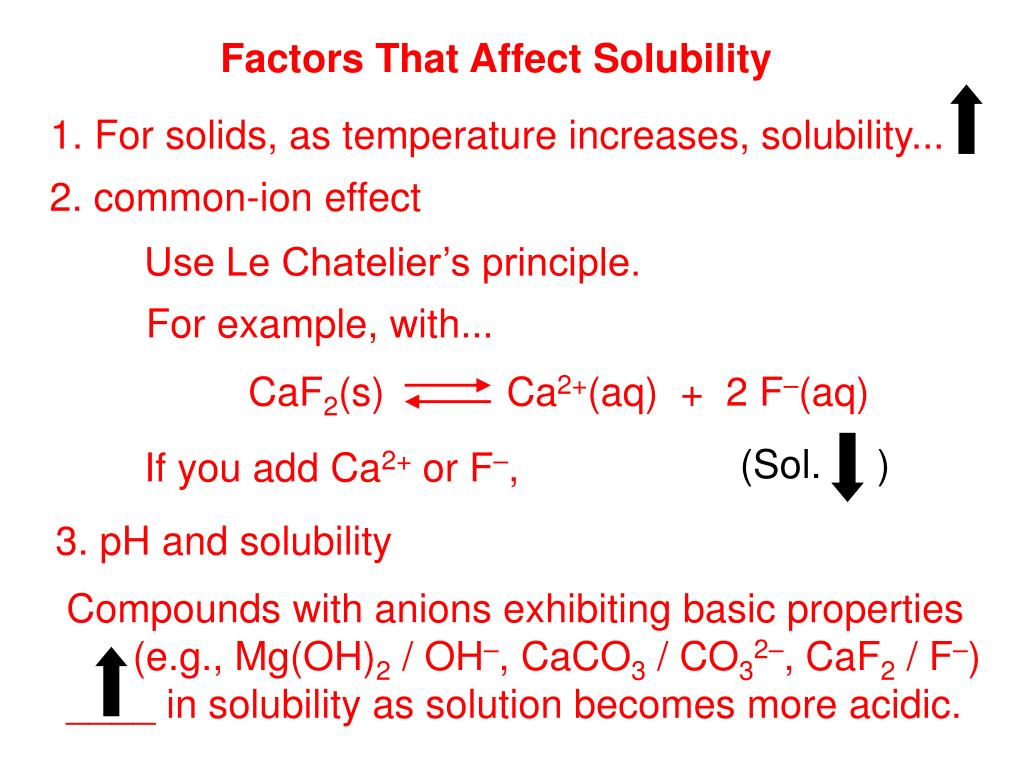
A salt can be either a strong electrolyte or a weak electrolyte. Salt: An ionic compound which consists of a cation other than H+ and anion other than OH.


 0 kommentar(er)
0 kommentar(er)
